About Us. |
Our Background and PhilosophySpectral Data Services, Inc. was founded in 1985, in order to provide rapid, first class NMR data acquisition capabilities and data analysis to industrial, university, and governmental clients who either do not have modern Fourier transform NMR spectrometers, or who are plagued by extremely long in-house turnaround times, or who require strict adherence to Federal regulations.
We have capabilities for all modern 1D and 2D experiments, as well as solid-state, liquid-state, and gas phase(e.g. ¹²⁹Xe) experiments. Spectra are typically recorded in less than 5 business days after sample receipt, and results are sent via FedEx or emailed as PDFs. For those requiring data in a hurry, our Spectral Express service typically provides 1 business day turnaround capability or 2 if QA review is required. Spectral Data Services, Inc. was established by Dr. Gary L. Turner, who is currently assisted by B.S. and M.S. level instrument operators and administrative staff. Gary has over twenty years of experience in solid- and liquid-state NMR, and is dedicated to solving your NMR needs. As required, additional consultants can be added to provide extra insight into solving your particular type of problem -- from patent disputes to difficulties with pilot plant runs. Data are collected under GLP (Good Laboratory Practice) and GMP (Good Manufacturing Practice) regulations, to insure quality raw data and proper documentation and validation. Our philosophy is simply to be the best, friendliest, most helpful, rapid turnaround NMR service available. We want to help solve your problems! |
Our ProjectsWe look forward to working with you on:
|
GLP and GMP Compliance
Spectral Data Services, Inc. is in full compliance with the GLP (Good Laboratory Practice) regulations mandated by the EPA (Federal Register 40 CFR part 160) and the FDA ( Federal Register 21 CFR part 58), and the GMP (Good Manufacturing Practice) regulations mandated by the FDA (Federal Register 21 CFR Part 211), and is registered with the FDA. We have a full-time, dedicated quality assurance specialist in-house to insure compliance and validation. Of course, our clients are welcome to perform their own audits of our facility.
In addition to providing NMR results with GLP/GMP compliance, we also can prepare protocols and study reports, provide method validations, and conduct audits and inspections.
We are also licensed with the Drug Enforcement Administration (DEA) as an analytical laboratory performing NMR analyses of controlled substances. The required security, personnel, and record keeping measures have been implemented, and the analytical staff has been trained in appropriate written procedures. With this registration, we are allowed to handle controlled substances under DEA Schedules II through V.
Links to GLP/GMP General Information Sites:
In addition to providing NMR results with GLP/GMP compliance, we also can prepare protocols and study reports, provide method validations, and conduct audits and inspections.
We are also licensed with the Drug Enforcement Administration (DEA) as an analytical laboratory performing NMR analyses of controlled substances. The required security, personnel, and record keeping measures have been implemented, and the analytical staff has been trained in appropriate written procedures. With this registration, we are allowed to handle controlled substances under DEA Schedules II through V.
Links to GLP/GMP General Information Sites:
Our Team
Gary Turner (sdsnmr@sdsnmr.com)
|
David Miller (david@sdsnmr.com)
|
Ted Turner (ted@sdsnmr.com)
|
Consultants
Professor Eric Oldfield, feh.scs.uiuc.edu/, received his B.Sc. degree in 1969 from the University of Bristol and his Ph.D. degree in 1972 from the University of Sheffield, in Great Britain. He currently is a member of the School of Chemical Sciences faculty at the University of Illinois. His research interests are in nuclear magnetic resonance spectroscopic studies in physical, inorganic and biological chemistry.
NMR Equipment
|
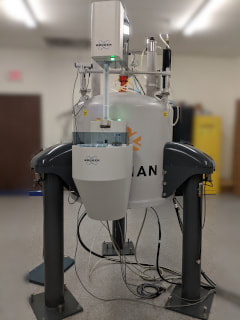
The 270 NMR System
Operating at a ¹H Larmor frequency of 270.620 MHz, is used for solid-state NMR (ssNMR) of dipolar nuclides (¹H, ¹³C, ²⁹Si, and ³¹P), using a 7mm CPMAS Doty probe. The 400-1 NMR System Operating at a ¹H Larmor frequency of 399.798 MHz, is equipped with 5 and 10 mm solution probes. It is a state-of-the-art Bruker TopSpin spectrometer, equipped with inverse-detection and PFG capabilities. Various nuclei include: ¹H, ¹³C, ¹⁵N, ³¹P, and ¹⁹F to name a few. Various tests include, 2D HSQC, COSY, HMBC, and DEPT. The 400-3 NMR System Operating at a ¹H Larmor frequency of 399.61 MHz, is equipped with a 5 mm solution probe. It is a state-of-the-art Bruker TopSpin spectrometer, equipped with inverse-detection and PFG capabilities. Various nuclei include: ¹H, ¹³C, ³¹P, and ¹⁹F to name a few. |
The 360-1 NMR System
Operating at a ¹H Larmor frequency of 363.335 MHz, is used for solid-state NMR (ssNMR). It is equipped with a 7mm CPMAS Doty probe and a 5 mm SuperSonic Doty probe. Various nuclei include: ¹³C, ¹⁵N, ³¹P, and ⁷Li to name a few. The 400-2 NMR System Operating at a ¹H Larmor frequency of 399.853 MHz, is equipped with 5 and 10 mm solution probes. It is a state-of-the-art Varian INOVA spectrometer, equipped with inverse-detection and PFG capabilities. Various nuclei include: ¹H, ¹³C, ³¹P, and ²⁹Si to name a few. The 400-4 NMR System Operating at a ¹H Larmor frequency of 399.665 MHz, is equipped with a 5 mm solution probe. It is a state-of-the-art Agilent (formerly Varian) MR-400 spectrometer, equipped with inverse-detection and PFG capabilities. Various nuclei include: ¹H, ¹³C, ³¹P, and ¹⁹F to name a few. |